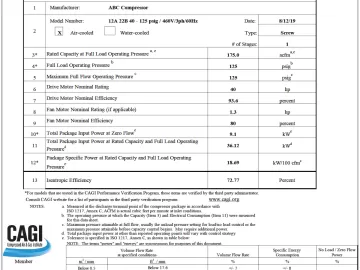
Profile: BeaconMedaes
In the U.S. as an example, the NFPA has taken the view that if your compressor draws in good clean ambient air, the air stays clean through the compressor, is then dried and filtered, when you deliver it to the patient it will be entirely satisfactory. After all, when you went into the hospital that’s what you were breathing and when you leave you will breathe it again!